The Anatomy Of An LED Bulb
Posted by Amit Soni on 14th Nov 2011
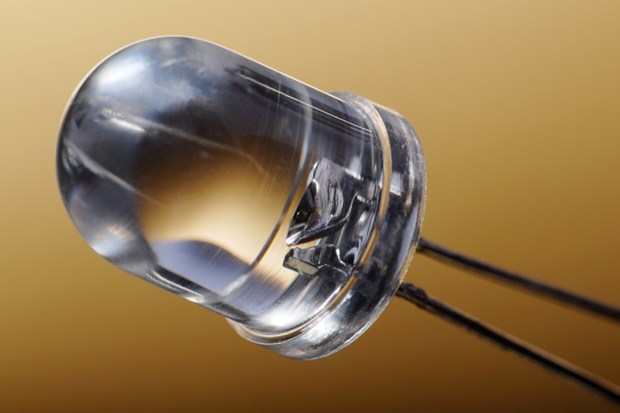 It’s all well and good using LED lights. More and more people are making the switch to LED lighting and taking advantage of the energy savings, longer lifespan and environmental benefits.
It’s all well and good using LED lights. More and more people are making the switch to LED lighting and taking advantage of the energy savings, longer lifespan and environmental benefits.
We all know about these things, but how does an LED actually work?
LEDs are a type of light source that do not rely on filaments, and unlike traditional light bulbs, can plug directly into an electrical circuit. It does this using a nitrous-based semiconductor material.
A semiconductor carries the same physical principles of both conductors, such as metal, and insulators, such as rubber. They can be made of either organic or inorganic material, though most today are inorganic.
Nearly all semiconductor material is made from groups of elements that are very close together in the periodic table, generally where the post-transition metals, metalloids and other non-metals are.
You’ll typically find semiconductors inside LEDs are made of AlGaInP (Aluminium, Gallium, Indium and Phosphorus) and GaInN (Gallium, Indium and Nitrogen), as they are widely used in lasers too.
These will either be single crystal (where atoms are arranged and repeated in an organised sequence), or amorphous (where atoms are arranged randomly). It probably isn’t surprising to know that amorphous compounds are easier to fabricate.
There are 2 bands inside a semiconductor that conduct electricity – the conduction band and the valence band. The conduction band carries negatively charged electrons, and the valence band carries positively charged protons, also known as holes.
Yet another name for these are n-type conductors for higher electron concentrations, and p-type conductors for higher proton concentrations.
A p-n junction is formed when an n-type material and p-type material come into contact. This junction is also called a diode, and forms the cornerstone of countless electronic devices, including LEDs – which remember is short for light emitting diode.
When a positive direct current is applied to the p-side, the electrons travel from the conduction band to the valence band and ‘fall into’ the holes. And in doing so, they release energy.
The most obvious kind of energy they release is photons, also known as light, and this is what you see when you switch the LED on. Other kinds of energy are released too but in far smaller amounts – such as heat.
While the science behind LED can leave you feeling like you’ve just sat in a physics class, the reasons why you should buy them are considerably straightforward in comparison.
The absence of a wasteful filament means that LED bulbs generate even more useful light at a fraction of the energy – drastically reducing the cost of running your household lighting.
Now that was a lot simpler wasn’t it?